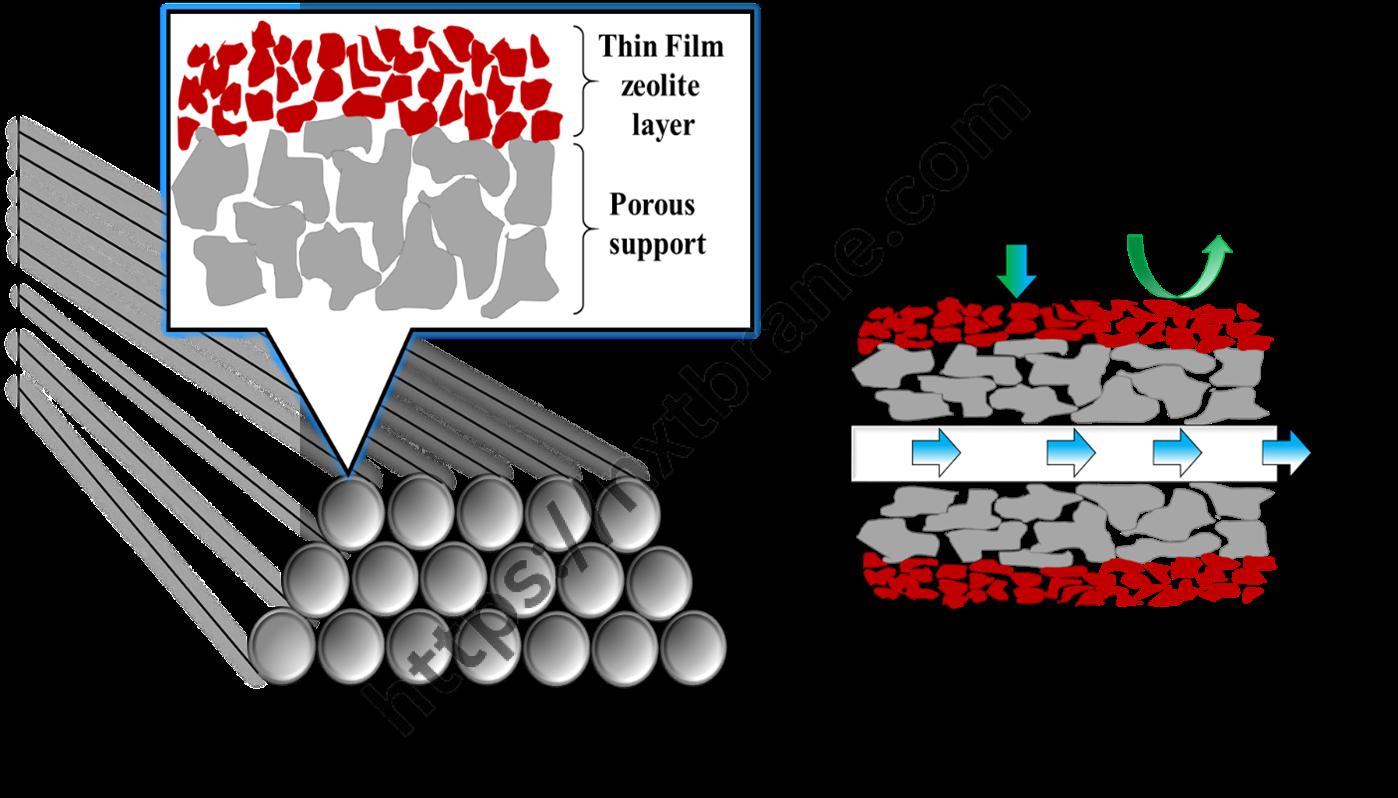
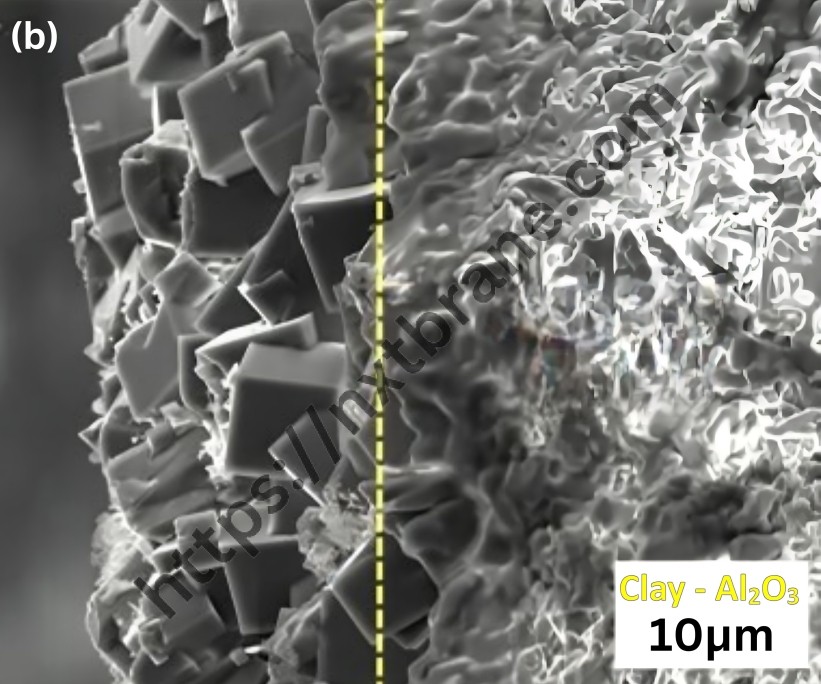
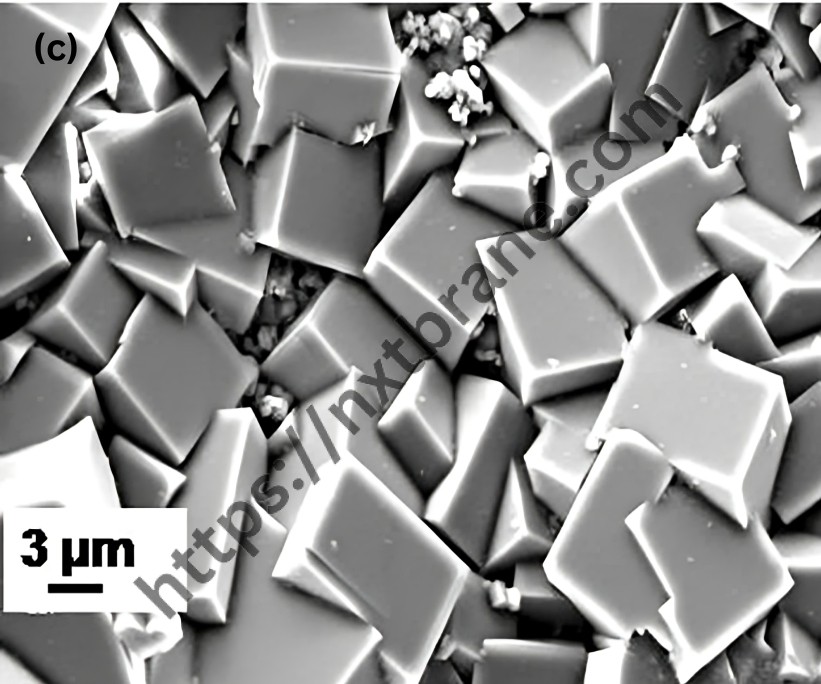
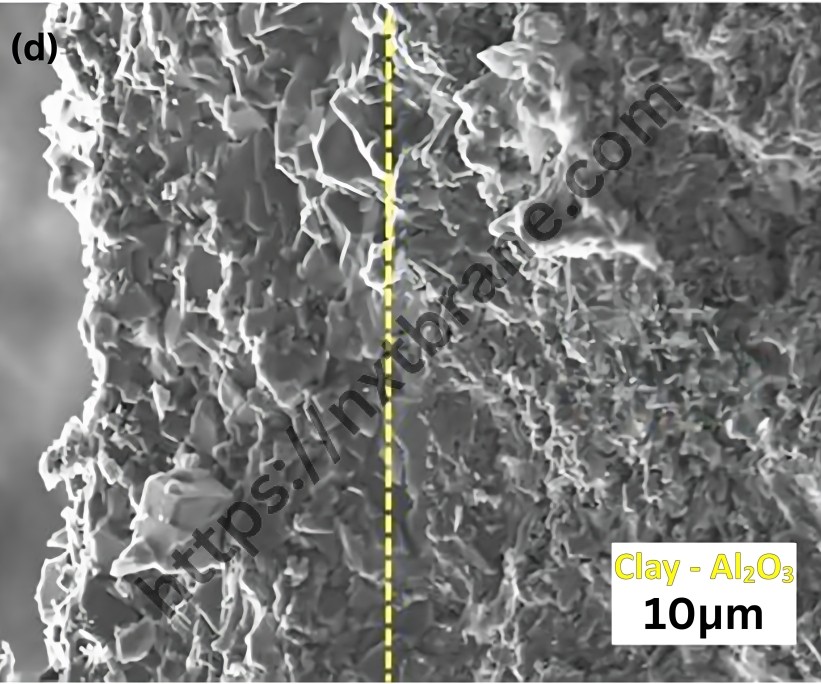
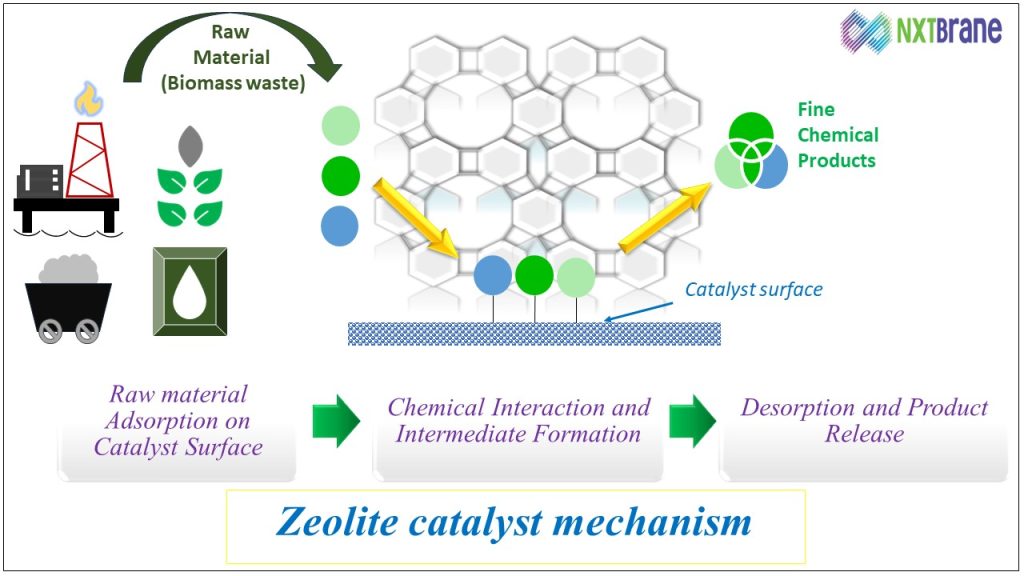
One of the most significant properties of zeolites is their catalytic activity. The porous structure of zeolites allows for the selective adsorption of molecules, making them ideal catalysts in various chemical reactions. The uniform pore size of zeolites can act as a molecular sieve, selectively allowing certain molecules to enter and react within their confined spaces.
Our Zeolite molecular sieves possess unique properties that facilitate various applications. They excel in liquid separation/purification (solvents, hydrocarbons, etc.), gas separation/purification (natural gas, air, etc.), moisture control, and chemical processes. Depending on the pore size, these molecular sieves fall into different available types such as 3A, 4A, and 5A, each with unique properties such as high adsorption capacity, thermal strength, compressive strength, and durability.
Zeolite molecular sieves, also known as 3A molecular sieves, are a specific type of crystalline aluminosilicate. These molecular sieves possess a regular micro pore structure with a pore size of about 3 angstroms (Å), making them highly effective for adsorption processes. They selectively adsorb molecules with diameters smaller than 3Å. These molecular sieves have a high adsorption capacity, compressive strength, durability, and a high adsorption flux.